log in
Active Users
Feedback
About and Contact
© 2020 DOUBTCOOL
Give reasons
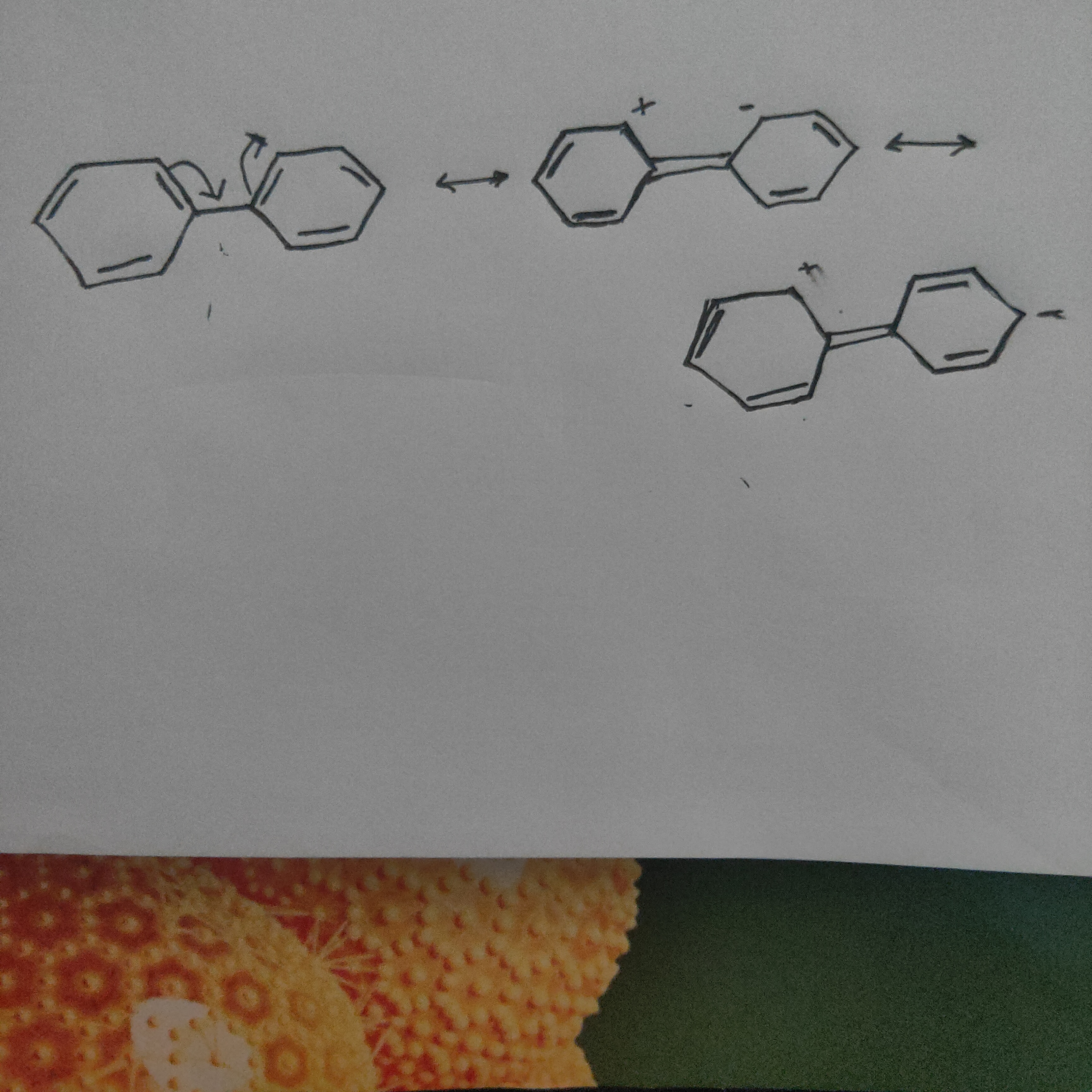
Phenyl group is known to exert negative inductive effect, but each phenyl ring in biphenyl (C6H5-C6H5) is more reactive
than benzene towards electrophilic substitution.
[JEE Mains 2018]
Phenyl group is known to exert negative inductive effect, but each phenyl ring in biphenyl (C6H5-C6H5) is more reactive
than benzene towards electrophilic substitution.
[JEE Mains 2018]
4 regular answers available
biphenyl, one phenyl group acts as
electron donor, while other acts as
electron acceptor. Thus, due to this
reason, the phenyl ring is more
reactive than benzene towards
electrophilic substitution
biphenyl, one phenyl group acts as
electron donor, while other acts as
electron acceptor. Thus, due to this
reason, the phenyl ring is more
reactive than benzene towards
electrophilic substitution
biphenyl, one phenyl group acts as
electron donor, while other acts as
electron acceptor. Thus, due to this
reason, the phenyl ring is more
reactive than benzene towards
electrophilic substitution